Preclinical Research Services
With over 30 years of experience in drug development, Altasciences offers specialized preclinical research services. Our expertise spans a wide range of in vivo non-GLP and GLP safety assessment solutions in both rodent and non-rodent species to thoroughly evaluate pharmaceuticals, biologics, animal health products, agrochemicals, and biocides. Our scientific and regulatory teams develop customized research programs and conduct individualized preclinical studies to ensure that your IND/NDA-enabling toxicology, safety pharmacology, and laboratory studies comply with global regulations.
Connect with our seasoned experts for tailored advice on your preclinical research needs.
EXCELLENCE IN PRECLINICAL
DRUG DEVELOPMENT SERVICES
As a leading CRO, we provide a suite of unparalleled services, designed to streamline your path to market. Our team of skilled scientists and experts ensures quality data for your critical decisions, advancing your lead compounds to first-in-human trials and accelerating your drug development milestones.
Gain comprehensive insight with our detailed fact sheets. Covering everything on preclinical drug development, these resources offer an in-depth look at our capabilities in small and large molecule research services.
PURPOSE-BUILT
FACILITIES
SAFETY STUDIES
COMPLETED ANNUALLY
TEAM MEMBERS
TAILORED FULL-SERVICE PRECLINICAL CRO SOLUTIONS
Discover our extensive range of customizable preclinical CRO services. Our full-service offering, from formulation to bioanalysis and beyond, is designed to meet your unique project needs with precision.
They include:
- formulation
- analytical chemistry
- bioanalysis
- PK/TK data analysis
- immunohistochemistry
- specialized necropsies
- anatomic pathology
- clinical pathology
- SEND (standard for the exchange of nonclinical data)
VERSATILE ANIMAL MODELS AND ADMINISTRATION ROUTES
Leverage our expertise in a broad spectrum of animal models and routes of administration. From rodents to nonhuman primates, and oral to dermal administration, we ensure meticulous studies for every requirement.SPECIES
- rats
- mice
- guinea pigs
- rabbits
- swine
- miniature swine
- dogs
- nonhuman primates
(Cynomolgus, Rhesus, others upon request)
ROUTES OF ADMINISTRATION
- oral
- parental
- infusion
- ocular
- dermal
- implant
- intravaginal and intrapenile
- rectal
PRECLINICAL STUDY TYPES
Working with a wide range of pharmaceutical companies from across the globe, our team of scientists and technicians has been conducting preclinical research for decades. Our safety testing services include the following study types:
-
 LEAD OPTIMIZATION STUDIES
LEAD OPTIMIZATION STUDIES -
 DOSE-RANGE FINDING STUDIES
DOSE-RANGE FINDING STUDIES -
 PRECLINICAL PHARMACOLOGY STUDIES
PRECLINICAL PHARMACOLOGY STUDIES -
 PHARMACOKINETICS/ PHARMACODYNAMICS STUDIES
PHARMACOKINETICS/ PHARMACODYNAMICS STUDIES -
 CELL AND GENE THERAPY DEVELOPMENT
CELL AND GENE THERAPY DEVELOPMENT -
 PIVOTAL TOXICOLOGY STUDIES (acute, sub-chronic, chronic, carcinogenicity)
PIVOTAL TOXICOLOGY STUDIES (acute, sub-chronic, chronic, carcinogenicity) -
 SAFETY PHARMACOLOGY STUDIES (CNS, cardiovascular, respiratory)
SAFETY PHARMACOLOGY STUDIES (CNS, cardiovascular, respiratory) -
 IMMUNOTOXICOLOGY/ IMMUNUNOLOGY STUDIES
IMMUNOTOXICOLOGY/ IMMUNUNOLOGY STUDIES
COMMITMENT TO ANIMAL WELFARE IN PRECLINICAL RESEARCH
At Altasciences, we prioritize animal welfare in our preclinical research. Our staff are extensively trained in laboratory animal care, focusing on humane treatment and environmental enrichment. Adhering to strict regulatory guidelines, we ensure compassion and sensitivity in every aspect of our research practices.
Our Commitment to Animal WelfareADVANCED PRECLINICAL RESEARCH FACILITIES
Altasciences' state-of-the-art preclinical research facilities, spanning over 585,000 square feet in strategically placed locations throughout North America, provide an ideal environment for comprehensive safety testing and bioanalysis in rodent and non-rodent species. Conducting over 700 safety studies annually, we offer a full range of in vivo GLP and non-GLP safety assessments for your drug substances. Our facilities are equipped to support pivotal toxicology, safety pharmacology, and laboratory solutions for small and large molecules, ensuring your submissions meet global regulatory requirements.
Explore Our Facilities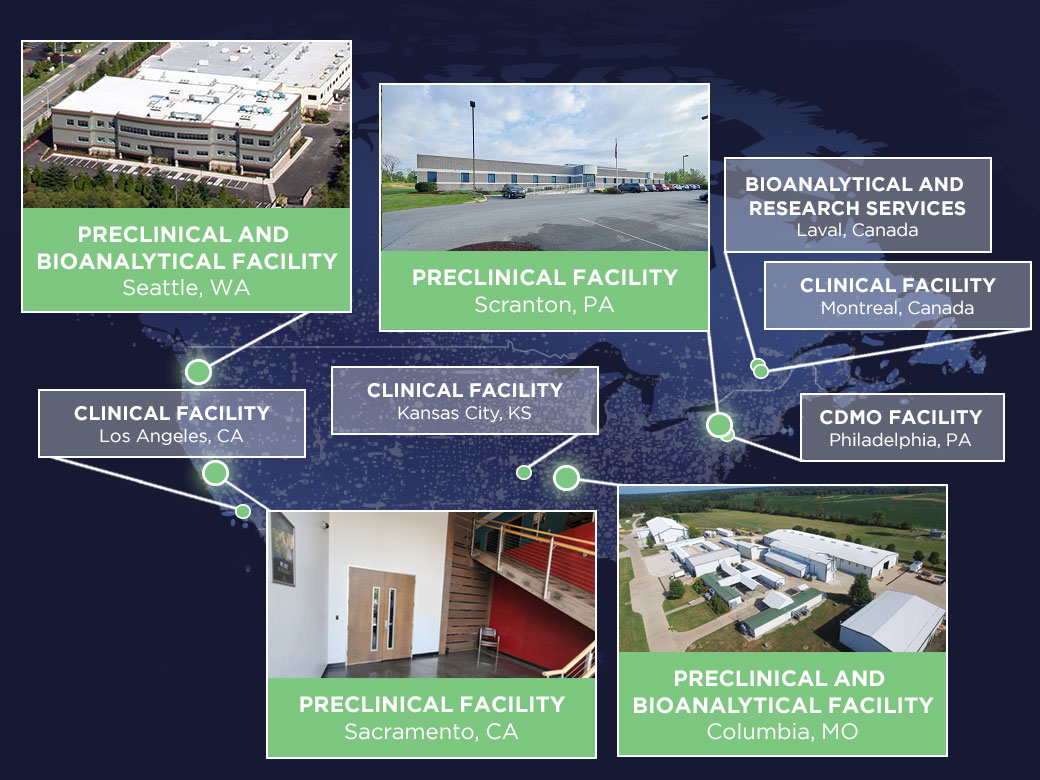
Related Resources
Consult this issue of The Altascientist to learn about key considerations when planning your preclinical assessment for IND submission.
Read this issue of The Altascientist to learn about nonclinical cell and gene therapy development and how to maximize translational opportunities to FIH trials.
Consult our fact sheet on Miniature Swine to see if they are a viable non-rodent option for your small or large molecule studies.
PRECLINICAL SERVICES - FAQs
WHICH PRECLINICAL DRUG DEVELOPMENT STUDIES DO YOU OFFER?
Our preclinical CRO services include single dose/acute, dose range finding, sub-chronic dosing, chronic dosing, carcinogenicity, safety pharmacology, toxicology, and PK/PD studies. Most safety assessment studies needed for your CTA/IND-enabling programs can be conducted at one of our many preclinical sites.
DO YOU OFFER SUPPORTING SERVICES FOR PRECLINICAL DRUG DEVELOPMENT?
We offer scientific, regulatory and strategic guidance, protocol development, project management, analytical chemistry, bioanalysis for small and large molecules, toxicokinetics, anatomic pathology, clinical pathology, reporting, SEND, and archiving of your preclinical research samples.
HOW DO YOU ENSURE THE 3RS ARE APPLIED WHEN CONDUCTING PRECLINICAL DRUG DEVELOPMENT STUDIES?
We have an innovative and robust animal welfare program that ensures that the physical and mental safety of our animal models is at the forefront of our activities, and that the 3Rs are rigorously applied. All employees sign a pledge to honor the animals in our care.
For a more in-depth look at how we ensure animals are treated humanely and with care, view our animal welfare and preclinical research videos.
DO YOU WORK WITH BOTH RODENTS AND NON-RODENTS FOR NONCLINICAL DRUG DEVELOPMENT PROGRAMS?
Yes, we work with both small and large animal models for all your preclinical research needs.
Our preclinical research facilities consist of 585,000 square feet of purpose-built space in four strategically placed locations throughout North America. They are equipped with animal rooms that include European housing, and we are constantly working to develop innovative low-stress methods of working with our research animals.
DO YOU OFFER PRECLINICAL TO CLINICAL INTEGRATED DEVELOPMENT PROGRAMS?
Yes, we do. When you partner with us for an integrated end-to-end program, we design clinical trials using the preclinical data to our best advantage. We ensure a constant flow of communication and open collaboration, so that you only have to Tell Us Once™, and we will take care of the rest.
Whether you choose our preclinical research services or the entire drug development solution from lead candidate selection to clinical proof-of-concept (and beyond), we are here to help.


