Preclinical Pharmacokinetic (PK) and Pharmacodynamic (PD) Studies
Preclinical PK/PD studies characterize exposure–response to inform dose selection and formulation before clinical trials.
Using a broad range of in vivo animal models, we conduct pharmacokinetic and pharmacodynamic (PK/PD) studies that deliver critical insights into your drug’s absorption, distribution, metabolism, and excretion (ADME). We offer real-time PK/PD data to support dosing strategies, optimize your drug’s performance, and reduce costly setbacks.
Whether you are developing a small or large molecule, our PK/PD studies provide the reliable and actionable findings you need to advance confidently toward regulatory approval and first-in-human clinical trials.
Optimize Your First-in-Human Trials with Predictive PK/PD Studies
Preclinical PK/PD studies in early-phase drug development explore how drug candidates interact with biological systems, providing critical insights before clinical trials begin.
Using data generated from these studies, our scientific experts can inform you on:
- Dosing strategy: using ADME studies, we determine the appropriate starting dose for humans.
- Efficacy signals: we correlate blood concentration with biological response to determine the therapeutic potential of the substance and revise the drug design, if needed.
- Route of administration: depending on factors like the drug's properties, the desired speed and location of its effect, and other factors, we establish the best route of administration (orally, nasally, intravenously, etc.).
- Formulation development and optimization: we refine the dosage form based on bioavailability and metabolism, to enhance delivery, efficacy, and stability.
- Regulatory compliance: PK/PD studies provide critical data to support regulatory submissions by demonstrating dosing strategies and pharmacologic effects—all key for approval to advance to clinical trials.
Our scientists work from four strategically positioned preclinical sites across North America, bringing their specialized expertise to the following areas:
- small and large molecules
- antibodies
- gene and stem cell therapies
- genome editing technologies
- nucleic acid therapeutics
- vaccines
Our PK/PD evaluations include:
- bioavailability and bioequivalence studies
- PK/PD modeling and simulations
- toxicokinetics for early safety assessments
Tailored Pharmacokinetic (PK) Studies
We conduct specialized PK studies to support your early-phase drug development―from discovery to IND submission.
Our preclinical PK studies provide vital insights into your drug’s behavior by assessing the pharmacokinetic profile of your new drug candidate and informing on the ADME characteristics of the substance in rodent and non-rodent animal models. This information helps determine your drug’s concentration in the bloodstream at various times, allowing our scientists to assess the safety and efficacy of the new therapy. We also develop thorough PK models and conduct simulations that help determine half-life, clearance pathways, bioavailability, and volume of distribution.
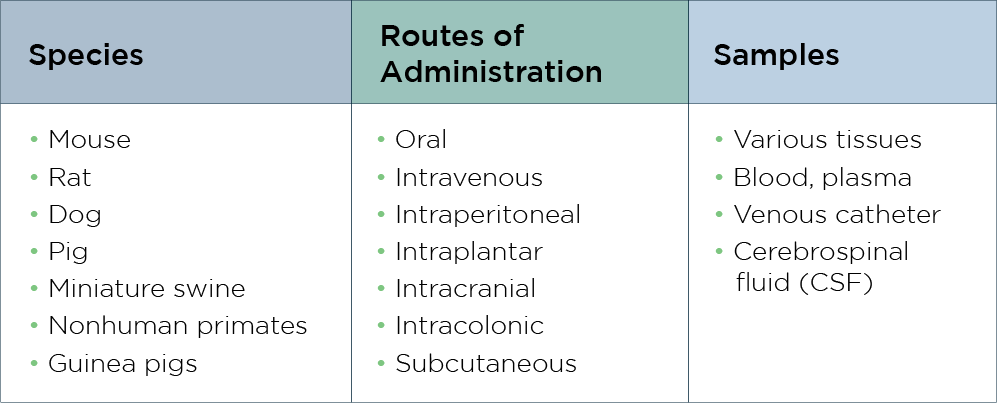
To optimize dosage techniques, we also perform complex population PK assessments using rich datasets, providing robust data for IND-enabling studies and human translation, and for evaluating exposure-response relationships in toxicology studies.
Our advanced PK study capabilities include surgical models, which are vital in generating reliable insights into drug behavior under clinically relevant conditions. Our advanced surgical techniques enable precise dosing, targeted delivery, and accurate sample collection—critical for high-quality PK studies. With a broad portfolio of surgical models, we help you evaluate drug effects on specific physiological systems more accurately. These clinically aligned methods enhance the translatability and validity of your preclinical data.
Pharmacodynamic (PD) Studies in Animal Models: Bridging Preclinical to Clinical
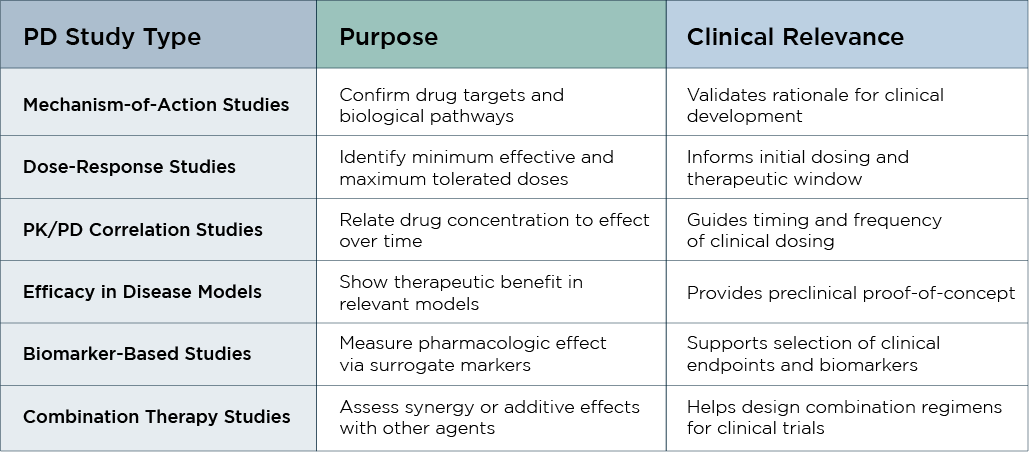
While PK studies focus on how a drug moves through the body over time, PD studies evaluate how your drug interacts with the body and produces a response, namely the therapeutic effects, mechanism of action, potency, and dose-response dynamics. These studies help bridge the gap between early discovery and first-in-human trials.
In preclinical PD studies, we also assess the pharmacological effect of your substance using cutting-edge approaches, such as biomarker analysis and sophisticated imaging tools. Our team creates personalized PD studies that complement your medication's unique target and therapeutic area.
Fully Integrated Bioanalytical Support
In preclinical PK/PD studies, robust bioanalytical support is critical for the accurate quantification of drug and biomarker levels across biological matrices. Our fully integrated PK/PD platform includes comprehensive bioanalytical capabilities, utilizing advanced methodologies and technologies to generate high-quality data that support regulatory requirements and informed decision-making throughout drug development.
Key Bioanalytical Services in Support of Your PK/PD Studies:
- Method development and validation
- Custom assay development for small molecules and biologics
- GLP-compliant method validation per global regulatory guidelines
- Quantitative analysis
- LC-MS/MS for quantifying small molecules in plasma, serum, tissue, and other matrices
- Ligand-binding assays (ELISA, MSD, flow cytometry, and more) for biologics and biomarkers
- qPCR and ddPCR for gene and cell therapy products
- Microsampling
- Translational biomarkers
- Immunogenicity testing
- Bioanalytical reports for IND/CTA submissions
Let’s Get Started
Preclinical PK/PD studies play a vital role in the drug development process, delivering key insights into your compound’s behavior and therapeutic potential. By addressing early uncertainties, we can help you gain a deep understanding of your drug’s safety, efficacy, and optimal dosing—laying a solid foundation before moving into clinical trials.
Ready to accelerate your path from discovery to clinic? Complete this form to connect with one of our experts.
Our Full Spectrum of Preclinical Research Services
Dive deeper into our extensive range of preclinical research services. From early-phase development to clinical trial readiness, our comprehensive offering ensures a seamless transition through each stage of drug development.
Pharmacokinetic (PK) and Pharmacodynamic (PD): FAQs
What is the difference between pharmacokinetic (PK) and pharmacodynamic (PD)?
Pharmacokinetics (PK) describes how the body affects a drug—including its absorption, distribution, metabolism, and excretion (ADME). PK is the study of drug concentrations over time to help determine how long a drug stays in the body, how it is processed, and its appropriate dose. Pharmacodynamics (PD), on the other hand, is a study that focuses on how the drug affects the body. It examines the drug’s mechanism of action, therapeutic effects, and the relationship between drug concentration and biological response. Together, these studies provide critical insight into a drug’s behavior and effects, helping researchers design safer, more effective treatments for a wide range of diseases.
What are pharmacokinetics (PK) and why is it critical to drug development?
Pharmacokinetics (PK) explains how a drug moves through the body—from the moment it is administered until it is fully eliminated. It involves four key processes: absorption, distribution, metabolism, and excretion. Together, these processes determine how much of the drug is available in the body at any given time and for how long. Pharmacokinetic studies play a central role in every stage of drug development, particularly in early preclinical stages, because they help determine the effective dose that stays within the therapeutic window. They support safety evaluations by showing how long a drug stays in circulation and where it accumulates, helping to predict and mitigate potential safety issues, such as drug accumulation or off-target effects. PK studies also guide dosing schedules and formulation development (for example, whether a drug should be immediate-release, sustained-release, or injectable). Whether a drug is taken once a day or multiple times depends on its PK profile. PK studies in animal models also help predict how a drug might behave in humans. This information is critical when selecting starting doses for first-in-human clinical trials. And finally, PK analysis is essential for regulatory submissions, to agencies like the FDA and EMA, as they require PK data to support Investigational New Drug (IND) applications.
How do pharmacokinetic (PK) studies optimize dosing regimens?
Pharmacokinetic (PK) studies play a vital role in optimizing dosing regimens by providing detailed insights into how a drug is absorbed, distributed, metabolized, and eliminated by the body. These data help define the therapeutic window—the range where a drug is both effective and safe—guiding the selection of appropriate dose amounts and dosing intervals. By understanding how long a drug stays in the system (half-life), how quickly it is cleared, and how much of it becomes available in circulation (bioavailability), scientists can determine how often the drug should be administered and by which route (oral, intravenous, etc.).
PK studies also support dose adjustments in special populations, such as those with impaired liver or kidney function, and enable personalized dosing strategies based on individual variability. When combined with pharmacodynamic (PD) data, PK modeling helps simulate different dosing scenarios, allowing researchers to identify regimens that maximize efficacy while minimizing side effects. This optimization is crucial for successful clinical development and regulatory approval.
How do PK/PD studies support regulatory approval of novel therapeutics?
Pharmacokinetic (PK) and pharmacodynamic (PD) studies provide a comprehensive understanding of a drug’s behavior and effects in the body. They are required to justify clinical development decisions and gain regulatory approval for novel therapeutics. PK/PD studies help determine the appropriate dose and dosing regimen, demonstrate proof of mechanism, and establish safety margins. These studies also support dose selection for first-in-human trials, help predict responses in special populations, and provide evidence needed to assess the benefit-risk profile of a new drug. Regulatory agencies rely on this information to evaluate a drug’s effectiveness, safety, and appropriate use, and it forms the basis for dosing recommendations in product labeling.
Related Resources
This webinar gives a comprehensive review of when and how to conduct cognitive and pharmacodynamic testing during first-in-human trials on CNS-active compounds.
Using case studies, this webinar highlights pitfalls that are common in the early stages of drug development programs, in order to help you avoid unnecessary delays and costly rework.
This webinar explores the critical role that safety pharmacology studies play in the drug approval process, as well as how to improve your cardiovascular, CNS, respiratory, and ion channel studies to avoid potentially catastrophic delays in your development timeline.
In this webinar you'll learn more about the flexible selection of pharmacodynamic measures to enhance the pharmacology, safety, and efficacy evaluation of a CNS-active drug in early clinical trials.
This issue of The Altascientist provides guidance on how to best plan your preclinical assessment for a successful regulatory submission.




