IMAGINE
PROACTIVE DRUG DEVELOPMENT…
WHAT IS PROACTIVE DRUG DEVELOPMENT?
Altasciences' integrated CRO/CDMO services and proactive approach translate into a results-driven exchange of information that reduces complexities, mitigates risk, condenses timelines, and enables cost savings for your entire drug development program or just one study.
Altasciences' Proactive Drug Development accelerates decision-making by offering expert guidance and synchronized early-phase services to reduce timelines by up to 40%. Our integrated solution drives success at each milestone with a tailored program that unites bioanalytical services, preclinical safety evaluation, formulation development, clinic-ready manufacturing, on-demand clinical pharmacy, and clinical testing to proof of concept, all within one organization. With drug development managed by one partner, activities can occur in parallel, rather than sequentially. Altasciences can support your entire drug development programs end to end, or you can partner with us for just one study — we offer you complete flexibility using the same approach.
Proactive Drug Development provides comprehensive communication plans and expertly designed roadmaps to get you to clinical proof of concept faster. Our unique organizational structure and integrated processes reinforce our ability to anticipate specific program needs. A centralized scheduling system facilities active timeline management and immediate responses. The synergistic relationship we develop with each client translates into a results-driven exchange of information that maximizes opportunities for success.
How Does Proactive Drug Development Work?
Altasciences provides you with clear, customized roadmaps, supported by real-time data generation, a proprietary communication platform, and central program management and scheduling.
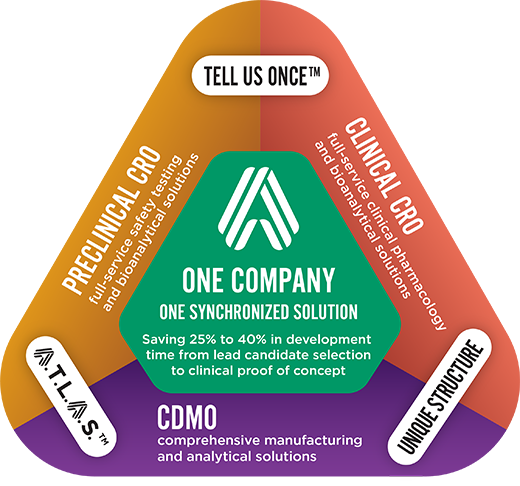
It's based on three core pillars:
- How we communicate – Tell Us Once™
- How we bring a project to life –

- How we organize ourselves – A unique organizational structure
How we Communicate
Tell Us Once™ is Altasciences’ commitment to communication. Ask Albert is the proprietary database behind the commitment, fueling integration and facilitating information-sharing across departments.
As a molecule advances, so does our clients’ information; we eliminate the need for clients to repeat themselves at each stage of the process. You only need to tell your story once, and we’ll take care of the rest.
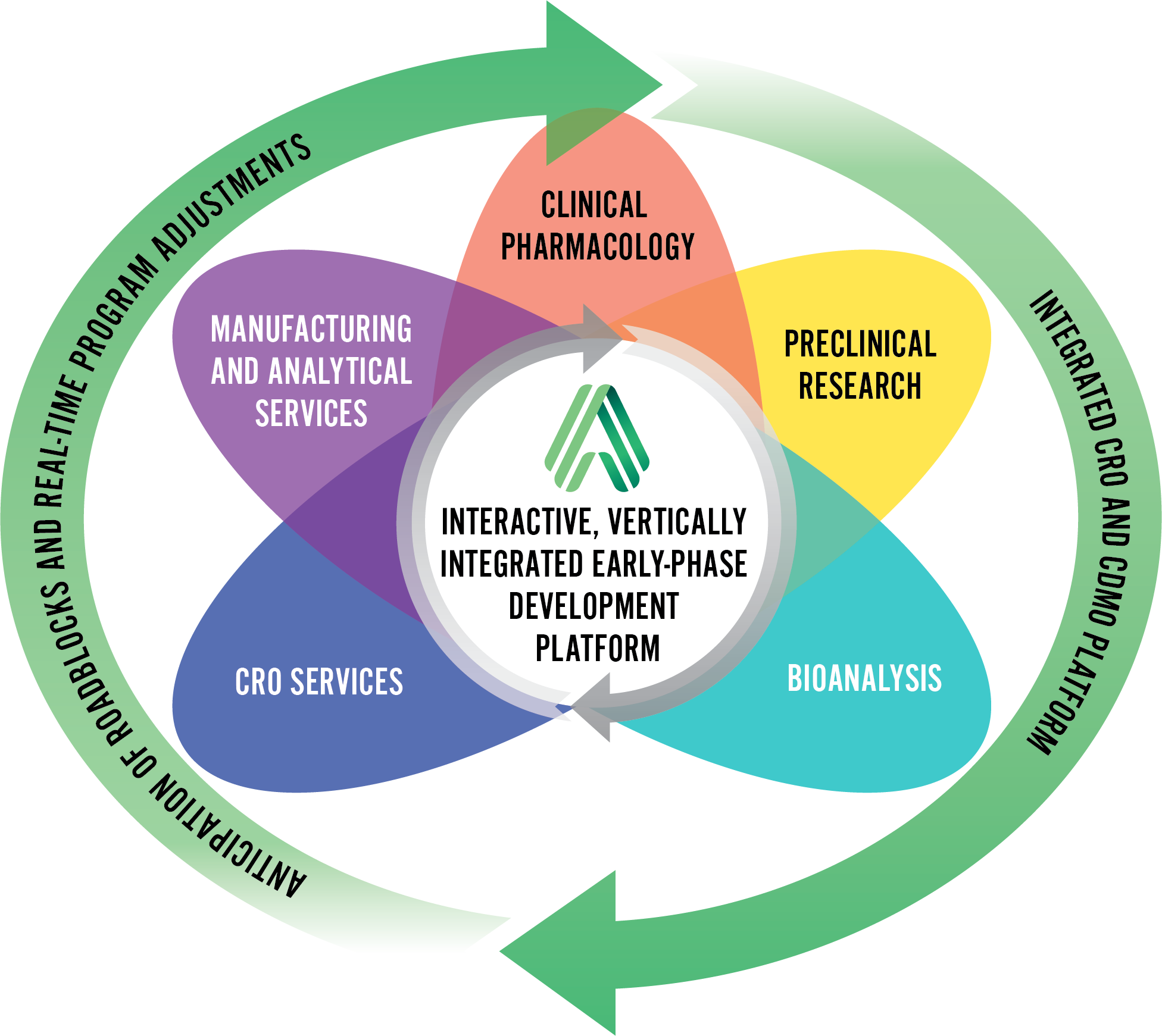
How we Bring a Project to Life
Using an A.T.L.A.S. approach, we anticipate and mitigate program-specific roadblocks, and streamline an integrated approach to CRO and CDMO services.
- Customized roadmap for each drug development program
- Dedicated cross-functional project manager
- Single centralized scheduling system
- Active timeline management
- Real-time responses and proactive solutions for any challenges that may arise
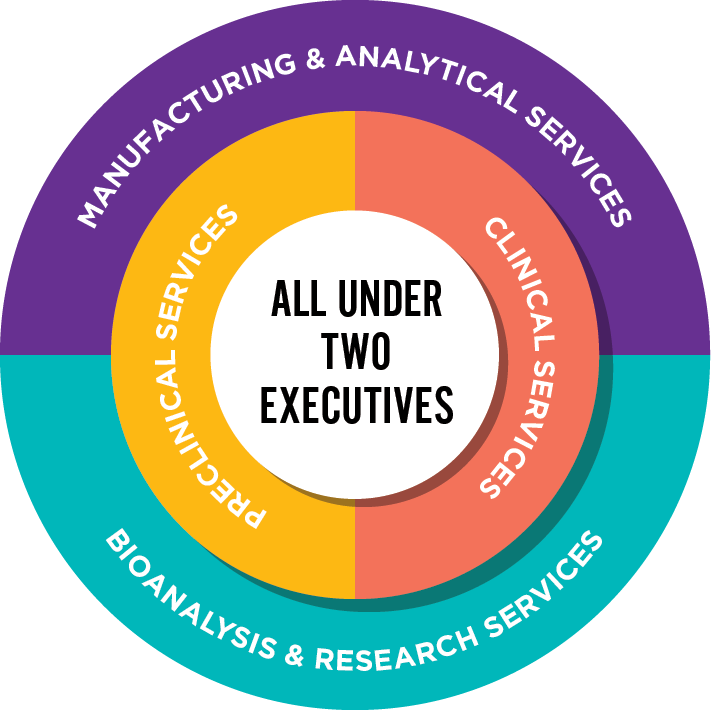
How we Organize Ourselves
Our unique structure — A grassroots level of integration, with two executives leading all scientific and operational teams, eliminating the internal silos that can impact your timelines. This group of forward-thinkers and scientific experts become extensions of your team, dedicated to advancing your studies.
At Altasciences, we do more than IMAGINE, we anticipate and deliver on the needs of your project from the start.