Preclinical Testing Facilities
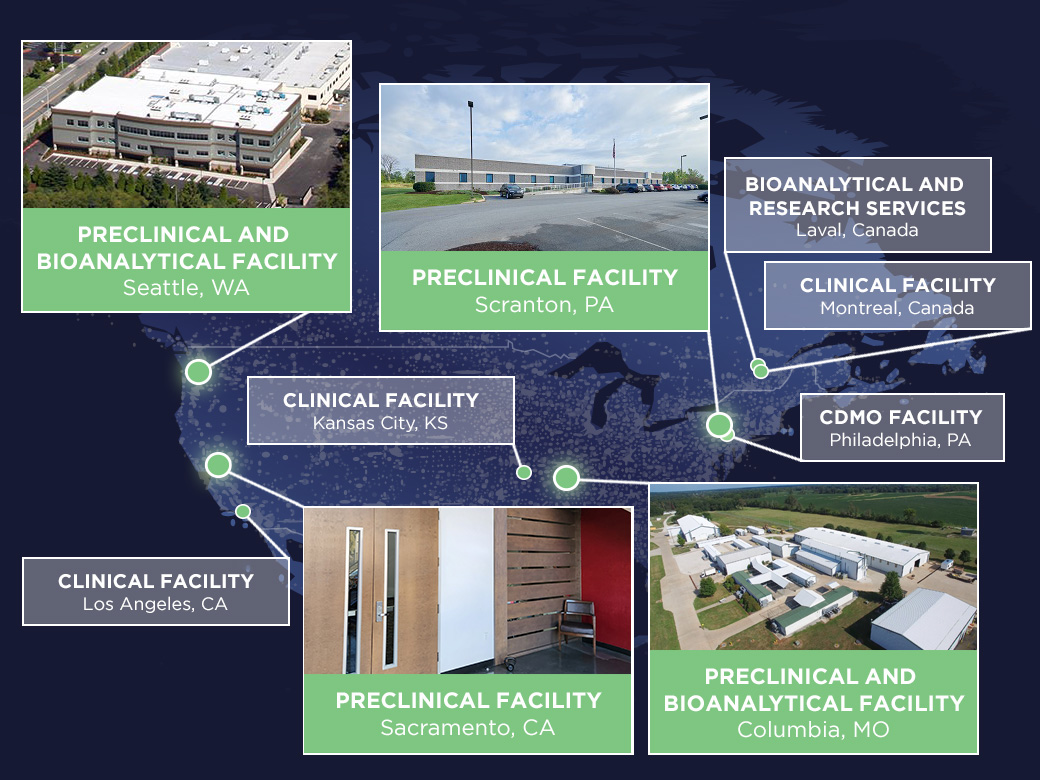
Our preclinical research facilities include 585,000 square feet of purpose-built space, where we conduct over 700 studies annually, offering a full range of in vivo GLP and non-GLP safety assessments in rodent and non-rodent species. We have four strategically placed sites in Seattle, WA, Scranton, PA, Columbia, MO, and Sacramento, CA, dedicated to comprehensive regulatory safety testing, bioanalysis, and other research support services for small and large molecules, including pivotal toxicology, safety pharmacology, and PK/PD studies, to meet global regulatory requirements for your IND submissions.
Connect with our seasoned experts for tailored advice on your preclinical research needs.
Our PRECLINICAL LOCATIONS
SEATTLE, WASHINGTON
- Conducted over 2,400 safety studies
- 440 staff members
- 160 custom-designed animal rooms, including European housing
- Capacity to house ~3,680 NHPs, ~500 canines, ~800 rabbits, and ~12,000 rodents
- On-site bioanalytical facility
- In-house bioanalytical laboratories to support internal and external studies
- In vivo non-GLP and GLP evaluation studies
- Large and small molecule safety studies
- Specialized in monoclonal antibodies, gene therapy, oligonucleotides, and ADC studies in NHPs
- On-site archive facility (~4,500 sq. ft.)
- Accreditations/Certifications:
- AAALAC accredited
- USDA registered
- OLAW assured
- BSL-2 certified
SCRANTON, PENNSYLVANIA
- 350-400 studies completed annually
- Over 85 staff members
- 40 animal rooms
- Capacity to house ~140 NHPs, ~350 canines, ~350 swine, ~6,500 rodents, and ~800 rabbits
- Small molecules and biologics
- IND-enabling toxicology and safety pharmacology, and chronic toxicity studies
- Specialized in ophthalmology studies
- On-site archive facility (1,840 sq. ft.)
- Accreditations/Certifications:
- AAALAC accredited
- USDA registered
- PHS Assured
- DEA Registered Schedules I-V
COLUMBIA, MISSOURI
- Over 180 studies completed annually
- Over 270 staff members
- 80 custom-designed animal rooms
- Capacity to house ~200 NHPs, ~700 canines, ~800 swine, ~2,300 rodents, ~300 guinea pigs, and ~100 rabbits
- Largest herds of miniature swine in the United States
- Small molecules, biologics and medical devices
- IND-enabling toxicology and safety pharmacology, and chronic toxicity studies
- Specialized in dermal studies
- Specialized in non-rodent surgery
- On-site archive facility (~4,000 sq. ft.)
- In-house bioanalytical laboratories to support internal and external studies
- Also supports clinical studies
- Accreditations/Certifications
- AAALAC accredited
- USDA registered
- PHS assured
- DEA Registered Schedules II-V
SACRAMENTO, CALIFORNIA
- Conducted over 140 studies
- 20 staff members
- 31,000 square-foot facility
- Specialized in large molecule non-GLP exploratory research
- Focused on ocular and CNS research in NHPs
- 18 animal rooms
- Capacity to house ~850 NHPs
- CDC quarantine area
- Accreditations/Certifications
- AAALAC
- USDA registered
- CDC registered
- OLAW assured