Cell and Gene Therapy Development
Altasciences provides comprehensive preclinical and bioanalytical solutions and extensive expertise to guide you through the complexities of gene therapy development. From preclinical safety studies to advanced bioanalytical services, we deliver the support needed to drive successful clinical outcomes. With you, we advance innovative treatments, providing hope for improved patient care and quality of life.
Take the first step towards advancing your gene therapy development. Contact our experts today to learn how our comprehensive services and tailored solutions can help bring your innovative treatments to life.
CELL AND GENE THERAPY EXPERTISE: ADDRESSING YOUR KEY QUESTIONS
At Altasciences, we understand the complexities and unique challenges of cell and gene therapy development. Our dedicated team delivers comprehensive solutions for your pharmacology, toxicology, and bioanalytical studies, ensuring your programs progress efficiently and effectively.
- In need of a partner with regulatory knowledge and expertise in evaluating cell and gene therapies' safety and efficacy?
- Looking for a reliable supply and availability of nonhuman primates (NHPs)?
- Looking for meticulously designed studies to assess safety, efficacy, biodistribution, and risks like germline integration?
- Need support for specialized routes of administration, such as intravenous, intrathecal, intravitreal, or suprachoroidal?
- Searching for specialized support for biodistribution studies and safety assessments tailored to your therapeutic area?
We have your gene therapy development covered.
Proven Track Record
In the last five years, we have completed 175+ in-life studies, over 95% of which involved NHPs. With over a decade of experience in IND-enabling studies for rare diseases and over five years working with CRISPR therapeutics in NHPs, we bring unparalleled expertise to your development programs. We will help you navigate the complex regulatory requirements and ensure the success of your IND submissions.
Supply and Availability of NHPs
At Altasciences, we eliminate sourcing delays through dedicated cynomolgus monkey supply agreements and a continuously maintained population of hundreds of naïve NHPs. Our vast pool of pre-screened NHPs for adeno-associated viruses (AAVs) ensures streamlined study initiation. Our four preclinical sites can start studies within eight to ten weeks of contract signing, so you stay on schedule.
Comprehensive Bioanalytical Capabilities
Our GLP-compliant Seattle lab delivers precise biodistribution analysis with on-site PCR capabilities for DNA and transgene RNA quantification. We also offer bioanalysis expertise for cell and gene therapy programs, including protein expression profiling, immunogenicity assessments, cellular immune response analysis, and robust method development and validation—all tailored to your unique therapeutic needs.
Cutting-Edge Operations
We specialize in complex routes of administration and support long-term studies, including work with infant/juvenile NHPs and specialized surgical biopsies. The experts at our purpose-built facilities in Sacramento (CA) support discovery, while specialists at our Seattle (WA) site focus on early development research for a wide range of cell and gene therapy platforms, including AAV, lentivirus, and CRISPR/Cas9 technologies.
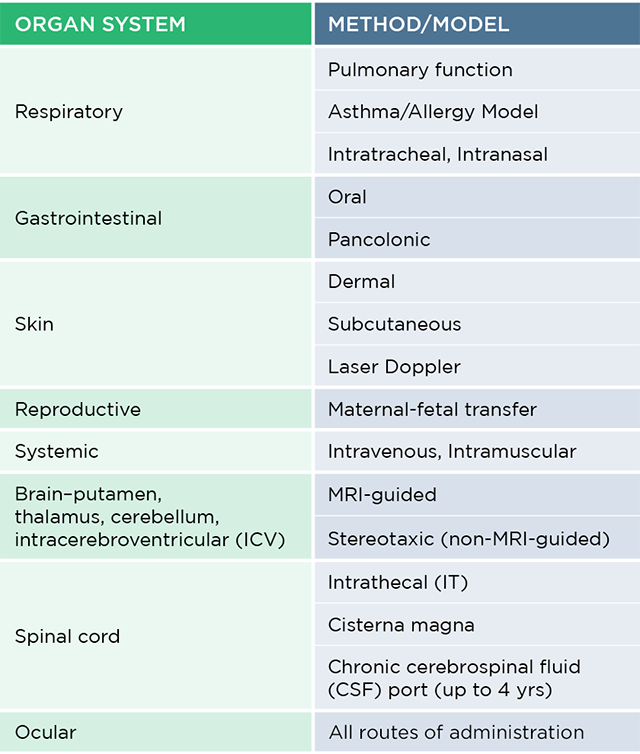
The table below illustrates various types of cell and gene therapy studies conducted at all our preclinical sites on vital organ systems, along with the methods or models employed for each.
The table below highlights the types of cell and gene therapy studies we conduct, covering vital organ systems and methods employed. Contact us to learn how our expertise can advance your programs.
Why Choose Altasciences—In a Nutshell
- Proven Track Record: 175+ successful in-life studies, extensive experience with NHPs, and expertise in CRISPR therapeutics.
- Comprehensive Capabilities: GLP-compliant labs for biodistribution analysis and bioanalytical support.
- Custom-Built Facilities: Purpose-built sites supporting innovative cell and gene therapy platforms.
KEY ELEMENTS TO CONSIDER FOR POTENTIAL GERMLINE INTEGRATION
We recognize the critical concerns surrounding the potential for germline integration in gene therapy research. Our approach addresses these concerns and ensures your studies are robust and compliant with regulatory expectations.

AAV PRE-SCREENING AVAILABLE AT ALTASCIENCES
Pre-existing immunity to AAVs can significantly impact the success of your gene therapy programs, as NHPs previously exposed to AAVs may have neutralizing antibodies that reduce the effectiveness of AAV-based therapies.
Given that AAV serotype 8 has the highest seropositivity rate—64% of 1,219 screened NHPs tested positive—it is crucial to screen animals for anti-AAV antibodies before administering gene therapy.
This pre-screening helps identify animals with high antibody levels that may be unsuitable for toxicology studies, ensuring more reliable and accurate results for your research.
Screening for Pre-Existing Antibodies to Adenovirus
Below are two assays available for pre-screening anti-AAV8 antibodies. These enable you to make informed decisions and optimize the success of your studies.
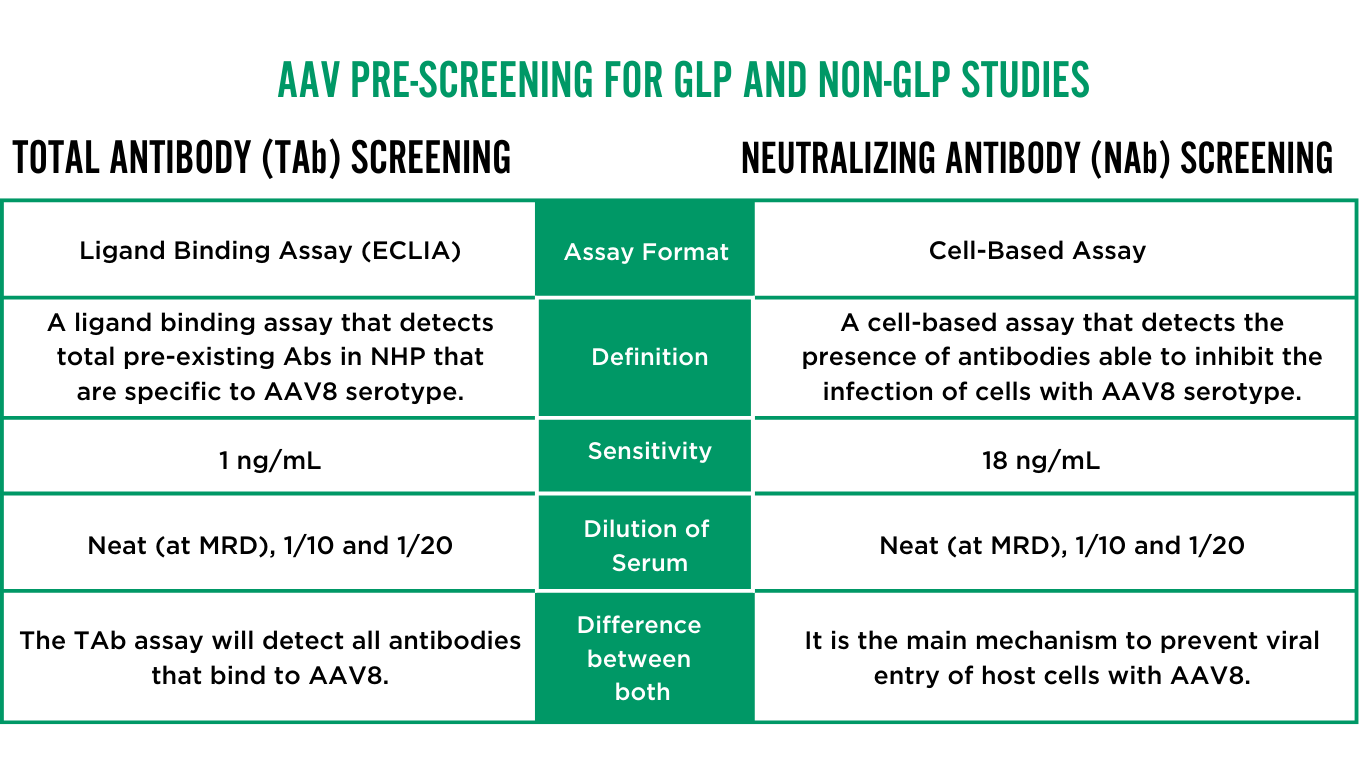
Achieve greater flexibility in your research with qualified methods that are suitable for both GLP and non-GLP studies. Our assays are adaptable to various serotypes and species and can be customized to meet your specific needs for in-study immunogenicity assessments. This flexibility ensures that you can tailor your approach to the unique requirements of each phase of your research, driving more reliable and impactful results.
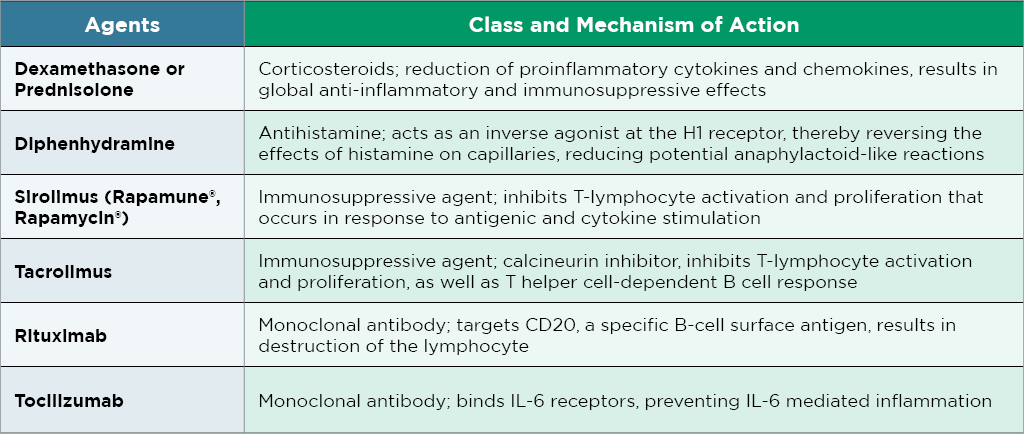
Immunomodulatory Approaches
Addressing immune responses is crucial in gene therapy, with immunosuppression and immunomodulation offering innovative strategies to optimize treatment outcomes.
We advise against using immunosuppression solely for biodistribution evaluation. However, in cases of species-specific transgene responses or heightened immune reactions, it can mitigate challenges and support smoother gene therapy development.
Alone: dexamethasone, prednisolone, diphenhydramine, or tocilizumab
As a combination:
- Prednisolone, diphenhydramine
- Tocilizumab, tacrolimus
- Prednisolone, rituximab, diphenhydramine, sirolimus