Clinical Trials
Early cardiac assessment studies to determine cardiac safety profile
|
||
|
February is Heart Month, a time to raise awareness on cardiovascular health. Cardiac safety issues are one of the main reasons promising drugs are halted in development. With the collaboration of a number of leading core ECG vendors and our in-house clinical units specially designed for assessing cardiac safety, Altasciences has designed and conducted over 30 early cardiac safety and QT prolongation studies. |
||
|
Whether you choose to conduct your cardiac assessment trials earlier (such as in first-in-human studies) or a little later (after completing Phase II), you can benefit from the following advantages:
|
||
|
In this issue of The Altascientist, we discuss the ICH regulations, and provide a thorough review of the design and timing of QT studies for your drug development program. Included is a recent case study on the QT assessment in a novel cannabis trial involving 80 subjects. |
||

|
Answers to your questions on the development of biologics and biosimilars
Artificial Intelligence Application in Early Phase Drug Development
Trends in Early Phase Drug Development
It’s the start of a new year, and our thoughts are turni
Clinical Trials for Metabolic Disorders— What You Need to Know

|
|
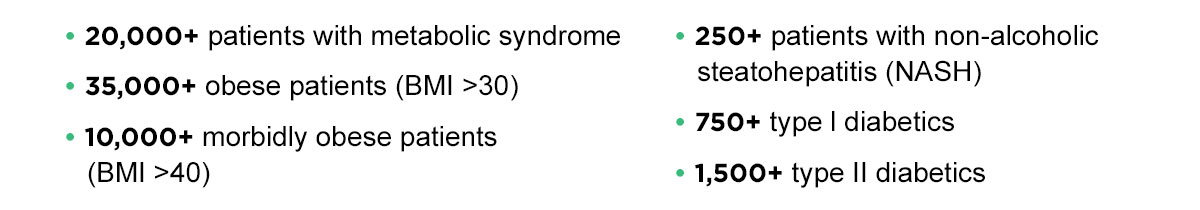
With over 25+ years of extensive experience conducting clinical trials and strategic alliances with a number of leading research centers, ,Altasciences is the best partner for studies requiring access to a large database of patients with metabolic disorders. We have completed over 50 studies to date. Our database of over 345,000 participants includes: |
|
|
In a recent NASH study, Altasciences had the best recruitment numbers out of 10 global sites recruiting for the study, even though we were included in the recruitment process only two months after the other sites. When the overall objective was met and recruitment was stopped, we had recruited more than 30% of the patients. We have much more to share, including a Q&A session on clinical trials for metabolic disorders with an expert in the field, our end-to-end recruitment process, and a case study on diabetes. |

|

|
C4X Discovery: Altasciences appointed by Indivior to conduct Phase I clinical trial with C4XD’s Orexin-1 for treatment of Opioid Use Disorder
9 December 2019 – C4X Discovery Holdings plc (AIM: C4XD), a pioneering Drug Discovery company, is pleased to note the announcement that Altasciences has been selected by Indivior PLC to conduct "A Phase I, Double-Blind, Placebo-Controlled, Randomized, Single Ascending Dose Study to Assess the Safety, Tolerability and Pharmacokinetics of INDV-2000 (C4X3256) Under Fasting and Fed Conditions in Healthy Volunteers”.
Laval, Quebec, December 9, 2019 – Altasciences has been selected by Indivior PLC to conduct "A Phase I, Double-Blind, Placebo-Controlled, Randomized, Single Ascending Dose Study to Assess the Safety, Tolerability and Pharmacokinetics of INDV-2000 (C4X_3256) Under Fasting and Fed Conditions in Healthy Volunteers", pursuant to the National Institutes of Health (NIH) Funding Opportunity Announcement RFA-DA-19-002, dedicated to the development of medications to prevent and treat opioid use disorder and overdose. The grant was made to Indivior by the NIH in fiscal year 2019, to apply scientific solutions to reverse the national opioid crisis. In March 2018, Indivior entered into a license agreement with C4X Discovery Holdings PLC (C4X) whereby Indivior obtained exclusive global rights to develop and commercialize INDV-2000 (C4X_3256).
Renal Impairment Study Designs Trends, Optimization, Adaptive Approach. A Review.
Drug-Drug Interactions - What you Should Know
the study of Drug-Drug Interactions
Tackling Challenges in First-In-Human Trials
Customized Solutions for your Renal and Hepatic Impaired Trials

|
|
Altasciences has designed and conducted a large number of renal and hepatic impaired studies and provides a flexible approach that allows for customization depending on your unique study needs. We partner with leading external sites to ensure that our renal and hepatic impaired patients have the specialized clinical oversight needed, and we leverage their databases to ensure we access the patient populations your studies require. |

|

|
Clinical Case Study - First in Human



