Will Biosimilars Replace Biologics?
Biologic drugs came on the market with the production of insulin in 1982. Since then, biologics have become the fastest-growing class of therapeutic compounds. They have provided treatment options for people who suffer from some of the most serious medical conditions. Thanks to biologics, patients with rheumatoid arthritis, cancer, rare blood disorders, multiple sclerosis, diabetes, and HIV/AIDS have better treatment options.
Over 620 biologics are licensed by the FDA. These drugs accounted for over $380 million in global sales in 2022, and demand is growing exponentially. This is due to the fact that they can have fewer side effects than broadly acting drugs. In addition, they have been successfully on some diseases not treatable by small molecule drugs.
Biosimilars are drugs that are very similar to currently approved biologics. With about 100 biologic patents expiring between 2022 and 2027, the emergence of biosimilars has been unsurprising. The global biosimilar market is expected to reach $44.7 billion USD by 2026. This is considerably up from its revenues of $15.6 billion USD in 2021.
But what exactly is a biosimilar? Is it what people call “generics”? And can it truly replace a biologic?
Biologics vs. Biosimilars
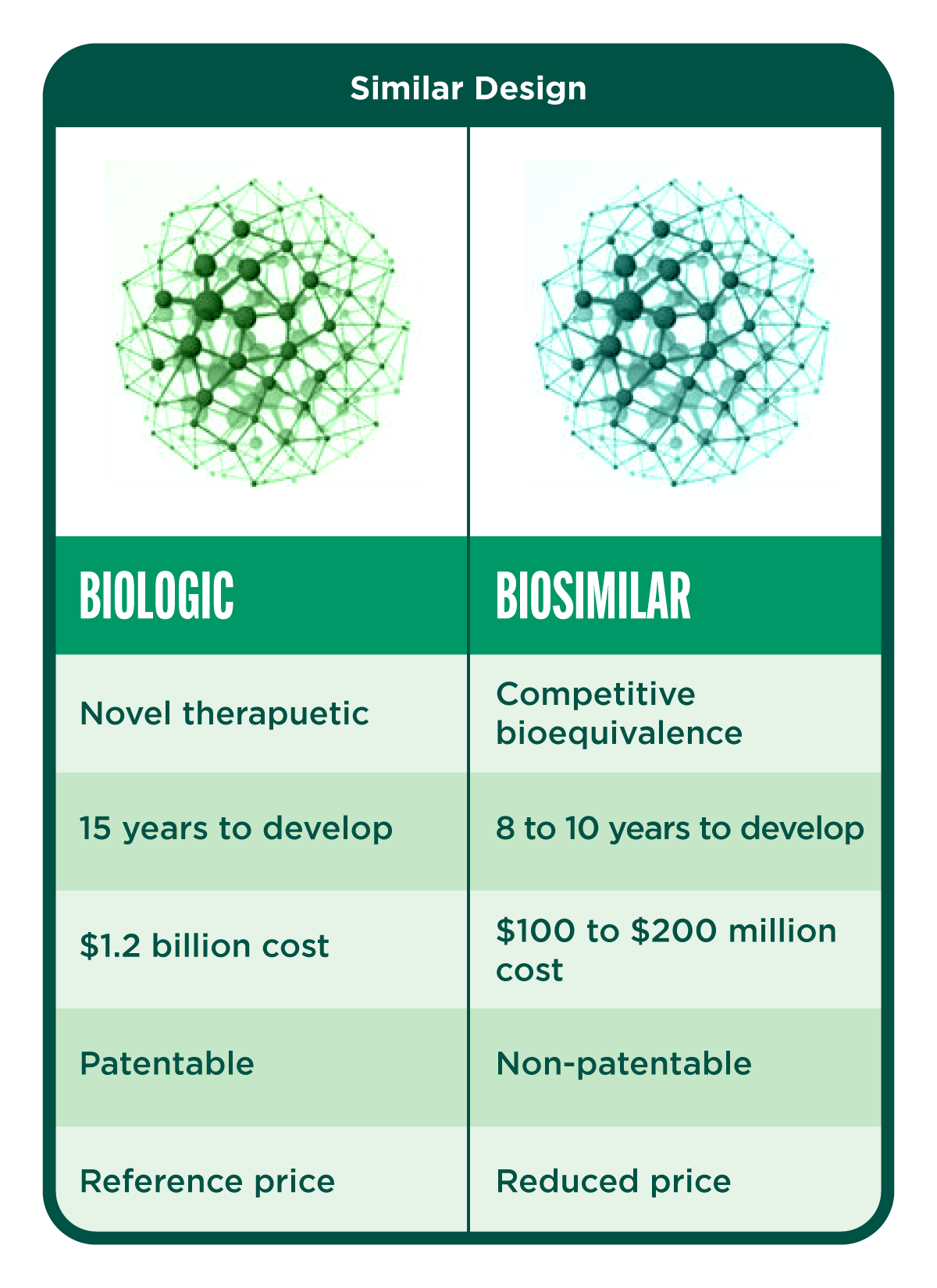
Biologic drugs are large, complex proteins made from living cells through highly complex manufacturing processes. While generic drugs are copies of chemical drugs, a biosimilar is similar, but not identical, to the original biologic medicine. A biosimilar enters the market following the patent expiration of an authorized biologic. It is approved only after being found to be highly similar to an approved biological product (the reference product). Important factors include safety, purity, and potency, and in some cases efficacy. Minor differences may be allowed. Biosimilars need to exhibit structural and functional similarities with comparable pharmacokinetic and pharmacodynamic properties to the reference product.
Is It Easier to Get Biosimilars Approved by Regulatory Agencies?
It can be easier to get a biosimilar approved by regulatory agencies than a biologic. When the patents of biologics started to expire in the 2000s, only a few biosimilars were approved due to vague product guidelines, regulatory uncertainties, and cautious physicians. However, with clearer guidelines now, the rate of biosimilar development and approval has accelerated. Regulatory agencies such as the European Medicines Association (EMA), the Food and Drug Administration (FDA), and Health Canada (HC) have developed strict regulatory guidelines for the evaluation and approval processes of biosimilars regarding their physical, chemical, and clinical traits. This has helped pave the way for rapid development and approval and increased market access and affordability. The EMA, FDA, and HC will only approve a biosimilar product if it has the same mechanism of action, route of administration, dosage form, and strength as the reference product.
Though manufacturers may intend to copy a biologic drug, a biosimilar with a different manufacturing process will never be identical to the reference product. This is because for many biologics, the posttranslational modifications make even the reference product a mix of compounds with slightly different structures. As a result, biosimilars go through a rigorous evaluation approval process to show that they are as safe and effective as the original biologic at treating the indicated diseases. The manufacturer also has to produce evidence regarding the quality control of the processes involved in the formulation. Once the biosimilar is approved, it needs to be monitored continuously to detect adverse events which may not be detectable during the development stage.
Are Biologics and Biosimilars Interchangeable?
Because the structural difference between biologics and biosimilars is so minimal, it becomes very easy to make the mistake of assuming they are identical. However, this does not make them interchangeable. Not all patients will react to a biosimilar in the same way as they do to the original biologic. Therefore, switching medications could potentially cause a change in a person’s condition. The FDA stresses the importance of naming and labeling the products with both their original brand name and the biosimilar “generic” name. This reduces risks and ensures the patient receives the drug intended by their physician. In this way, the source of any adverse events can be accurately traced.
Is Cost Driving Choice?
Biosimilars are often given the same indications as the originator drugs in order to reduce the development cost, hence the market price, making it more accessible and affordable to patients. As more biosimilars roll out into the market, the savings patients have from the reduced costs are very large. If patients have the same treatment success with the biosimilar, why pay the higher price for the reference drug?
What Does the Future Hold for Biosimilars?
There is little doubt that biosimilars will gain further traction in the coming years. Already, biosimilars have been developed for blockbuster biologics like Lantus, Rituxan, Herceptin, Remicade, Enbrel, Neulasta, Avastin, and Humira. However, barriers to competition for biosimilar companies include high manufacturing costs, complexity of production, and unclear regulatory policies involved in the development of these drugs.
As the biosimilar pipeline continues to grow, it will be interesting to monitor and see if the number of emerging biosimilars will truly surpass the number of new biologics coming into the market.
You may also be interested in:
The Altascientist: Issue 4—Key Considerations for Biosimilar Clinical Pharmacology Studies
Fact Sheet: Biologics/Biosimilars



