Miniature Swine: Changing the Bias for Nonclinical Studies on Small Molecules and Biologics
Miniature swine have been widely used for research studies since the 1960s—most commonly within the scope of dermal testing due to similarities of their integument to humans.
Recently, the increase of historical data and wider regulatory acceptance has revealed that the miniature swine model is also an excellent choice for a wide range of nonclinical studies beyond dermal research. Their physiological and anatomical similarities to humans make them valuable animal models for studying various diseases, drug efficacy, and safety. Their cardiovascular, gastrointestinal, respiratory, and immune systems closely resemble those of humans, allowing researchers to conduct studies that more accurately predict human responses to drugs and treatments. Furthermore, miniature swine provides a more cost-effective and practical alternative while offering high translational relevance.
Addressing the Bias of Using Minipigs
As with all safety assessment, the most appropriate animal model is dependent on the needs of each project. In the past, biopharmaceutical companies have overlooked incorporating miniature swine into their species selection process—seemingly due solely to the historical preference of using dogs.
Previously, one obstacle to using swine in nonclinical studies has been size, which, despite the term “minipig” can still grow substantially—typically weighing between 35 to 45 kg at maturity. But that has now changed as U.S.-based breeder of research swine, Sinclair Bio Resources, has developed a downsized miniature swine called the Sinclair Nanopig™, that is not only similar in size to a beagle (approximately 10 to 12 kg at maturity), but is also lower in terms of cost.
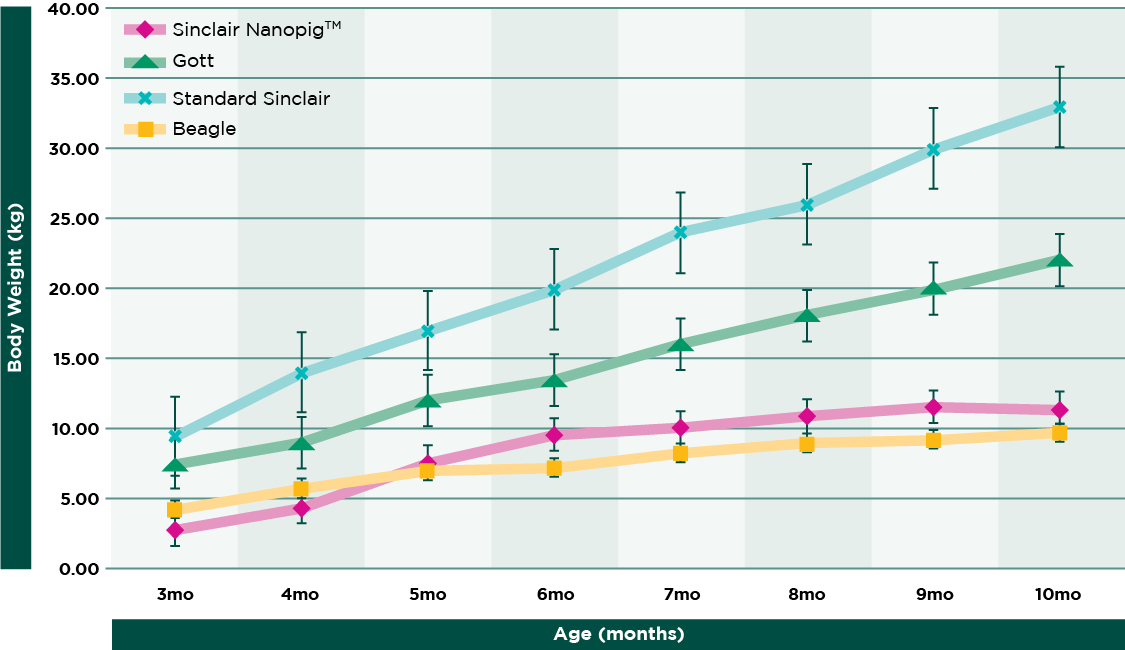
Miniature Swine as a Viable Option for Nonclinical Safety Studies
In a publication for the 12th annual miniature swine research forum, delegates from the forum stated, “There are no regulatory hurdles for using miniature swine, as the use is accepted by regulatory authorities all over the world, including Japan, Korea, China, India, the EU, and the U.S. Nobody had experience with, nor knowledge of, any instances where the miniature swine had not been accepted by regulatory agencies”.
Animal models for each toxicology program are determined by examining several factors. In the case of UK-based pharmaceutical company GSK, each molecule is scientifically investigated, measured, and documented against these factors, and NHPs are only used if and when the swine or beagle do not meet the necessary requirements1.
For small molecule programs, the primary driver for non-rodent selection is metabolism and absorption—the two main components of which are cytochromes and transporters—and have been both found to have 70% similarity between swine and humans. Outside of the liver, the gastrointestinal tract has the most metabolic activity, and again, the physiology of the miniature swine is remarkably similar to that of a human, when considering the pH of the stomach, salivary amylase, and rate of gastric emptying to name a few.
Additionally, the industry's understanding of the binding affinity of miniature swine Fc gamma receptors to human immunoglobulins is increasing—especially as it becomes an integral part of the decision-making process when determining the most appropriate non-rodent species for human-relevant toxicity studies with a particular molecule.
Other Variables to Consider When Choosing Minipigs for Nonclinical Studies
The reasons that make miniature swine such as the Sinclair™ and the Göttingen™ (the most widely-used breed of miniature swine since the ‘90s) a viable option are detailed in this issue of The Altascientist, which highlights examples and provides real-world case studies to further demonstrate the advantages in miniature swine as an efficient animal model.
In this video, Scott Boley, Senior Scientific Advisor at Altasciences, explains the benefits of considering the minipig, such as:
- FDA-recommended
- docile species
- low cost
- large blood volume
- minimal zoonoses
- fast reproductive cycle
- rapid sexual maturity
- readily available stock
Although the miniature swine is not as commonly used in studies as other non-rodent species, its utilization has been steadily increasing as biotech and pharmaceutical companies recognize their potential. With greater characterization of the model and databases for historic control growing, miniature swine is in the perfect position to replace standard non-rodent species in many development programs.
Experts in Miniature Swine
Having conducted over 300 studies with miniature swine, the team of scientists and veterinary surgeons at our facility in Columbia, Missouri, have extensive knowledge and experience in GLP and non-GLP studies with small and large molecules using a range of miniature swine breeds. The facility houses one of the largest miniature swine populations in the U.S., and available breeds such as the Hanford™, Göttingen™, Sinclair Nanopig™, and the Yucatan™ are often used in a wide variety of therapeutic indications, including inflammation, oncology, CNS disorders, HIV, and dermal.
Visit our miniature swine or preclinical services webpages to learn more about our safety testing solutions.



