Exploring the Advantages of Canada for Early-Phase Clinical Trials
Where you decide to conduct your early-phase clinical trials could have a significant impact on your costs and timelines. There are lots of factors to consider when making that decision—in some cases, performing clinical trials in Canada and the U.S. simultaneously can be the right step towards optimizing your program. But as sure as maple syrup is great on pancakes, and mustard is better on hot dogs, some things are simply just a better fit.
In this blog, we will be addressing some of the reasons why conducting your early-phase clinical trials in Canada can be a great option, including:
- faster regulatory pathway
- diverse study participants
- tax incentives and funding
HOW DOES CANADA COMPARE TO THE U.S. AND EUROPE FOR EARLY-PHASE CLINICAL RESEARCH?
There are, of course, plenty of advantages to performing early-phase clinical trials in the U.S. and Europe: The research infrastructure supporting clinical research includes a well-developed network of CROs, clinical trial management systems, and other resources—not to mention that the U.S. is often at the forefront of innovation when it comes to new scientific research, second only to China. Yet, as of March 2023, studies show that 3.1% of clinical trials initiated worldwide in 2022 were running in Canada, with between 64% and 76% being industry-sponsored every quarter since 2018.
Both Canada and the EU use Clinical Trial Applications (CTAs) to authorize (or deny) the conduct of a clinical trial in their respective regions; in the U.S., this process is called an Investigational New Drug (IND) application. INDs for the FDA and CTAs for the European Medicines Agency (EMA) are not only more stringent than Health Canada’s—requiring more documentation such as clinical labels, chemistry, manufacturing, and control (CMC) information, on top of language and translation requirements for the EU—but are also lengthier processes overall. Both also require annual reports, with the EMA necessitating an annual development safety update report (DSUR)—which Health Canada’s does not.
Canada is compliant with the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), and home to many reputable clinical research teams, experienced in managing complex international studies. Health Canada, one of five federal agencies within a “health portfolio”, is overseen by Canada’s Minister of Health. This agency assesses clinical trial protocols to evaluate participation protection and safety, reviews drug quality, verifies principal investigator qualifications, and assures various committee reviews; all to an incredibly high standard.
SWIFT REGULATORY PATHWAY FOR EARLY-PHASE CLINICAL RESEARCH
Health Canada offers a seven-day review option for eligible comparative single-dose bioavailability/bioequivalence studies, and a 30-day default for a Clinical Trial Application (CTA), which is an easier to build, less demanding package that can save cost and time. For first-in-human (FIH) trials, the Research Ethics Board (REB) review process usually takes approximately one week.

This streamlined CTA gives Canada an edge over the U.S. and European processes, with the latter’s Part I and II CTA review timeline taking a minimum of sixty days (without any issues or changes)—with standard response times for extensions being twelve days. This can make all the difference to the timelines of your early-phase clinical trials, as can the CTA and REB’s clear, predictable, and transparent guidelines more efficiently facilitate the study design and execution of your clinical studies. They can also be used to support submissions for Investigational New Drug (IND) applications to the FDA in the U.S., enabling a smoother transition to late-phase clinical trials.
The data generated through CTA-approved trials is accepted in submissions for marketing authorization requests around the world in regions such as the EU and the UK, Asia, Brazil, and other ICH-compliant countries, positioning the CTA as an asset. In a previous blog article, we wrote an in-depth overview of Health Canada's 30-day regulatory review process, which you can read here.
DIVERSE CANADIAN POPULATIONS FOR EARLY-PHASE CLINICAL TRIALS
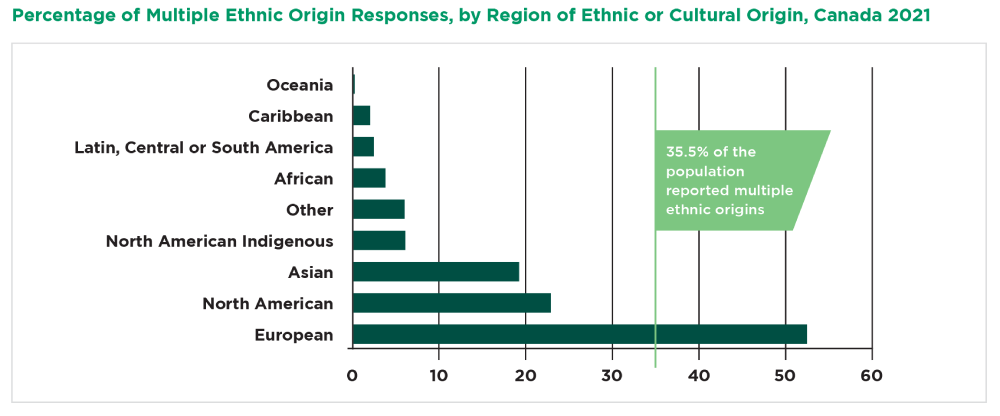
Canada is known for having an ethnically diverse patient population, and holds the highest percentage of foreign-born citizens among all of the G7 countries, with the 2021 census revealing 23% of the total population to be immigrants, in comparison to the U.S’. 13.6%. Statistics Canada reports that 35.5% of the population reported multiple ethnic origins. This means clinical trials can easily include representation from many of the targeted patient populations.
Canadian participants are also supported by Medicare, a publicly funded, and free, healthcare system, backed by the Canadian government (and managed by each province individually). This continuum of care philosophy has allowed pharmaceutical and biotechnology communities to utilize the exceptional health of study participants for their early-phase clinical trials.
The Canadian population is highly concentrated in the urban areas where research clinics are located, which facilitates recruitment efforts, and increases the chances of having a full panel in place, on time, to start key trials. Affordable, comprehensive public transit which provides easy access to the clinical pharmacology units facilitates patient/healthy participant screening and return visits.
CANADA'S COST-EFFECTIVE EDGE IN EARLY-PHASE CLINICAL TRIALS
Conducting your clinical trials in Canada can offer significant cost savings. The Canadian research environment operates at basically the same level of technological and scientific advancement as the U.S., but with a lower currency value that can benefit those looking to invest in clinical research.
Other tax incentives, such as the Scientific Research and Experimental Development (SR&ED) tax incentives, and the provincial and territorial research and development (R&D) tax credits, can also contribute to the cost-effectiveness of your development program—as can funding programs like the Strategic Innovation Fund (SIF).
WHO IS CONDUCTING EARLY-PHASE CLINICAL RESEARCH IN CANADA?
In March, 2023, AstraZeneca announced plans to expand its research operations to Ontario, Canada, creating a new rare disease development hub (rare diseases make up 30 to 40% of the clinical trials performed in Canada).
Other companies, such as Pfizer, Johnson & Johnson, Novartis, and Roche, are also capitalizing on Canada’s clinical trial advantages, with each having facilities based in Canada; Moderna also recently announced their first manufacturing facility outside the U.S., in Laval, Québec—just a few doors down from Altasciences’ headquarters and bioanalytical site. According to McDougall Scientific, Canada has the second lowest cost for managing clinical trials among the G7 nations, so it’s no surprise that many high-profile companies are using Canada for their operations. 
At Altasciences, our experts can help you prepare and submit your CTA and IND applications, support you during pre-CTA meetings, handle IRB submissions, and coordinate with regulatory authorities. We provide strategic guidance with international regulatory pathways, like those mentioned in this article, and others like the FDA, TPD, TGA, and ANVISA.
For more information, read or listen to Issue 31 of The Altascientist, which provides an unabridged look at the process, or watch our webinar presented by Altasciences’ Ingrid Holmes, Vice President of Global Clinical Operations, and Dr. Beatrice Setnik, Chief Scientific Officer, in which they take a deep dive into the benefits of choosing Canada for your next early-phase clinical trial.



