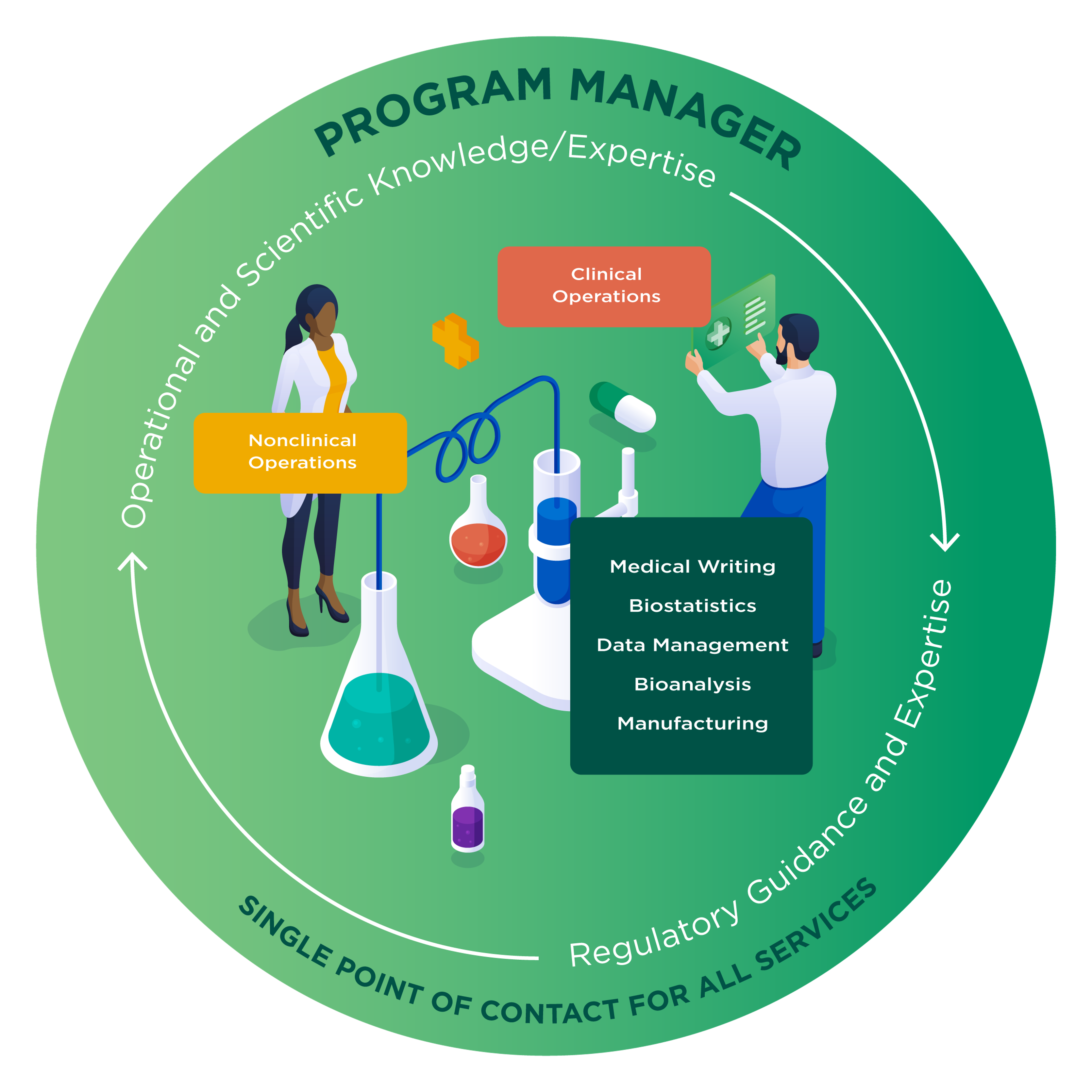
Program Management for One Point of Contact
With Altasciences, your program is managed by a single, cross-functional program manager, dedicated to your studies.
Our team seamlessly advances your molecule from preclinical testing to early-phase clinical studies, with a tailored, proactive approach that unites bioanalytical services, preclinical safety evaluation, formulation development, clinic-ready manufacturing, and clinical testing to proof of concept.
With a dedicated program manager overseeing your drug development program, our scientific and operational teams across sites collaborate efficiently, allowing study activities to occur in parallel where possible to save time and costs.
Access our fact sheet for more information on our unique Program Management offering.End-to-End Drug Development: Simplified and Efficient
Your dedicated, cross-functional program manager:
- Provides you with a centralized point of contact to improve speed, efficiency and communication.
- Manages your study timelines proactively using our proprietary scheduling system.
- Leverages our team of experts to review your emerging data and make strategic study design or timeline adjustments while continuously supporting your needs.
- Responds to your program challenges with solutions, in real time.
- Shares all your information across departments proactively, so you only have to Tell Us Once™.
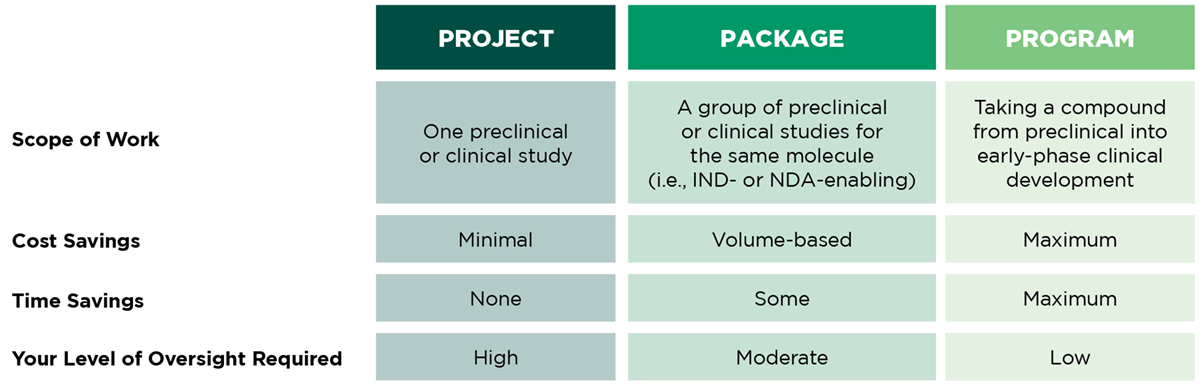
Shifting to a Program Model
Our program management model enables a seamless drug development experience with a single CRO/CDMO, increasing efficiencies for your entire program.
EXPLORE MORE OF ALTASCIENCES’ CRO SERVICES
Advancing your drug candidate smoothly to proof of concept is what we do best.